
Intellectual Property & Patents Part 2: Navigating a Safe Passage
Intellectual Property (IP) is the most valuable asset of information-age companies. Defining and protecting the value of that asset can be as important as creating it.
By Paulo Fontes, MD, FACS
TAKEAWAY: IP is your most important asset: The investment in time, resources and expertise required for effective IP management should be viewed not as a burden, but as an essential component of business strategy. Don’t Panic, you’ve got this.
As the primary mechanism for protecting innovation, a well-executed IP strategy—particularly through patent protection—proves essential for securing investment, enabling strategic licensing opportunities and maintaining competitive advantage throughout the commercialization process. This strategic foundation becomes particularly critical during due diligence processes while moving towards successful commercialization of assets.
The strength of a patent portfolio begins with comprehensive, unpublished experimental data that substantiates claimed innovations. In the complex landscape of biotechnology, where the path from discovery to commercialization is particularly intricate, the quality and depth of supporting data play a pivotal role in determining patent strength. Critical experimental protocols and methodologies must thoroughly support composition of matter claims, while reproducible results should clearly demonstrate practical utility. Furthermore, comparative data establishing technological advantages and specific evidence of therapeutic or diagnostic applications serve to reinforce the patent’s value proposition.
The distinction between patent applications and scientific publications represents a crucial consideration for biotech entrepreneurs. While both documents may cover similar scientific ground, they serve fundamentally different purposes and require distinct approaches. Scientific publications aim to advance knowledge through peer-reviewed sharing of research findings—emphasizing experimental results, data interpretation and theoretical implications. Their structure follows established academic formats, allowing for speculative discussion and theoretical exploration.
Patent applications, conversely, function as legal documents designed to secure proprietary rights. They demand precise, unambiguous language that clearly defines the invention’s boundaries and scope of protection. Where academic papers might broadly explore implications, patent applications must provide concrete, enabling descriptions that allow skilled practitioners to reproduce the invention without undue experimentation. This fundamental legal standard differs markedly from academic publication requirements, necessitating careful consideration of timing and disclosure strategies.
In the realm of patent applications, two primary pathways exist. The Provisional Patent Application (PPA) serves as an initial filing mechanism that secures a priority date and enables “Patent Pending” designation. While a PPA must include a detailed description of the invention, it does not require formal claims, inventor declarations or data. This provisional filing must be converted to a non-provisional application within 12 months. The non-provisional patent application represents a formal submission that undergoes USPTO examination and must satisfy all statutory requirements to potentially result in an issued patent. Another common type of patent filing at this time point is to file a Patent Cooperation Treaty (PCT) application that benefits from the PPA filing date and serves as a placeholder application for future filings in most foreign countries. These foreign filings from a PCT are referred to as nation stage entry filings and can be done in all countries that are signatories to the Patent Cooperation Treaty.
Of paramount importance in biotech patent strategy is the pursuit of composition of matter claims, which provide the broadest possible protection by covering the actual chemical or biological substances themselves, regardless of production method or use. These claims effectively prevent unauthorized making, using, selling or importing of the protected substances. However, a truly comprehensive patent strategy should extend beyond composition of matter to encompass method of use claims, formulation claims and process claims—creating multiple layers of protection for the innovation.
The Freedom to Operate (FTO) analysis stands as a cornerstone of successful IP strategy implementation. This comprehensive assessment is often conducted concurrent with patent filing to ensure the company can operate without legal impediments. A thorough FTO analysis serves multiple critical functions: It identifies potential infringement risks by uncovering existing patents that could pose legal challenges; it enables strategic planning by mapping the IP landscape and identifying viable innovation pathways; and it supports due diligence processes by demonstrating rigorous risk management to potential investors and partners. Early identification of these risks allows companies to modify product designs or negotiate licenses with patent holders, thereby avoiding costly legal disputes and potential delays in bringing products to market.
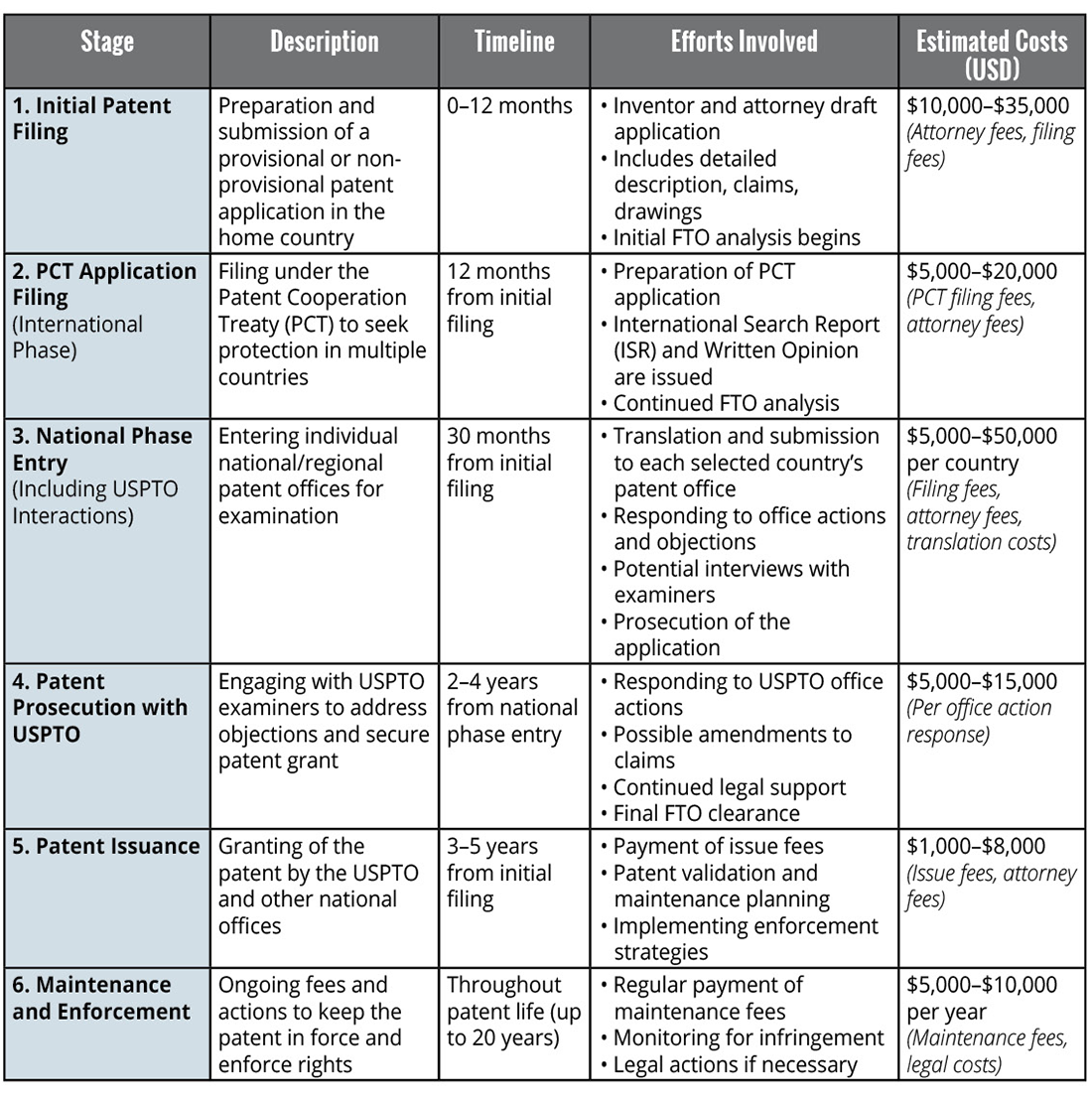
The patent filing process requires substantial investment across multiple stages. Initial filing costs typically range in the tens of thousands or more, encompassing patent preparation and preliminary FTO analysis. The PCT Application phase, facilitating international protection, requires an additional investment of $5,000 to $20,000. Globally, national phase entry patent filings can represent a significant escalation in cost, potentially reaching $20,000 to $50,000 per country, covering translation requirements and jurisdiction-specific submissions. Patent prosecution, involving interactions with USPTO examiners and potential claim amendments, may incur costs of $5,000 to $15,000 per office action. Ongoing maintenance and enforcement expenses typically range from $5,000 to $10,000 annually. It’s not unusual for a company to spend hundreds of thousands of dollars over a 3-4 year period to achieve full patent issuance.
Success in navigating this complex landscape requires engagement with specialized IP legal firms staffed by dual-degree lawyers holding both PhD and JD credentials. These professionals combine deep scientific understanding with legal expertise—essential for effective USPTO interactions and international patent prosecution. The investment in time, expertise and financial resources should be viewed not as a burden but as an essential component of building sustainable competitive advantage.
The implementation of a comprehensive IP protection program represents a fundamental investment in a biotech startup’s future. Success demands careful attention to legal requirements, strategic timing of disclosures, and thorough protection of innovations through well-crafted patent applications. While the investment required is substantial, it should be viewed as essential to long-term value creation and competitive advantage in the biotechnology sector. Most importantly, the early and ongoing execution of thorough FTO analyses provides the foundation for sustainable growth while minimizing legal risks that could otherwise derail the company’s progress. Regular portfolio review and adjustment ensure alignment between IP strategy and evolving business objectives, enabling companies to maintain their strategic position while identifying new opportunities for innovation and protection.
Progressive Stages of Patent Filing: Timeline, Efforts and Costs
The patenting process is a multi-stage journey that involves strategic decision-making, considerable effort, and financial investment. The table below outlines the key stages from the initial filing to the final patent issuance, focusing on the United States Patent and Trademark Office (USPTO) procedures. It can take years and cost hundreds of thousands of dollars.
Lessons Learned
Securing robust patent protection based on solid data and specific claims is foundational for the success of biotech startups. This is a time when resourceful scientific language needs to be translated in a timely manner into precise legal documentation. It not only safeguards the company’s innovations but also enhances its attractiveness to investors and partners. Concurrently, conducting a thorough FTO analysis is vital to navigate the competitive landscape without infringing on existing patents. This is probably one of the most important strategic developments for new companies to avoid future litigation. Understanding and effectively managing the complex patent filing process, while maintaining strategic flexibility, enables startups to maximize the value of their intellectual assets and achieve their business objectives. Biotech companies must work diligently with renown IP legal firms that have been working within this space for several years. These firms are staffed by dual degree lawyers who are knowledgeable of the scientific matters and fluent on the legal terms required for proper filings. The long-term interactions with the USPTO and the PCT filings requires moderate financial resources and intense intellectual input from the senior management team.
The investment in time, resources, and expertise required for effective IP management should be viewed not as a burden but as an essential component of business strategy. In the dynamic biotechnology sector—where innovation drives value creation—a well-executed IP strategy can provide the competitive advantage necessary for long-term success.
REFERENCES
- Lievrouw, Leah A. “Biotechnology, intellectual property, and the prospects for scientific communication.” Biotechnology and Communication. Routledge, 2004. 161-188.
- Kesselheim, Aaron S., and Jerry Avorn. “University-based science and biotechnology products: defining the boundaries of intellectual property.” JAMA 293.7 (2005): 850-854.
- Brower A. Lack of intellectual property protection worldwide threatens US. Biotechs. Biotechnol Healthc. 2006 Feb;3(1):21-2. PMID: 23424332; PMCID: PMC3571038.
- Rimmer, Matthew. Intellectual property and biotechnology: biological inventions. Edward Elgar Publishing, 2008
- C.B. Hing, D.L. Back, A review of intellectual property rights in biotechnology, The Surgeon, Volume 7, Issue 4, 2009, Pages 228-231, ISSN 1479-666X, https://doi.org/10.1016/S1479-666X(09)80090-5.
- Giugni D, Giugni V. Intellectual Property: a powerful tool to develop biotech research. Microb Biotechnol. 2010 Sep;3(5):493-506. doi: 10.1111/j.1751-7915.2010.00172.x.
- Singh, Kshitij Kumar. Biotechnology and intellectual property rights: legal and social implications. Springer, 2014.
- Paul, Matthew J., Harry Thangaraj, and Julian K‐C. Ma. “Commercialization of new biotechnology: a systematic review of 16 commercial case studies in a novel manufacturing sector.” Plant biotechnology journal 13.8 (2015): 1209-1220.
- Nambisan, Padma. An introduction to ethical, safety and intellectual property rights issues in biotechnology. Academic Press, 2017.
- Matthews, Duncan, and Herbert Zech, eds. Research handbook on intellectual property and the life sciences. Edward Elgar Publishing, 2017.
- Dutfield, Graham. Intellectual property rights and the life science industries: A twentieth century history. Routledge, 2017.
- Kiskis, Mindaugas. Intellectual Property Challenges for the Modern Biotechnology Enterprise: An Overview. Journal of Commercial Biotechnology; 2017, London 23:1, DOI:10.5912/jcb767.
- Toma, Antonio, Giustina Secundo, and Giuseppina Passiante. “Open innovation and intellectual property strategies: Empirical evidence from a bio-pharmaceutical case study.” Business Process Management Journal, 2018, 24.2: 501-516.
- Noonan, Kevin E. and Torrance, Andrew W. Biotechnology Patent Law Top Ten of 2018 Broad Wins, Sovereignty Loses, and Patent Dance,” Akron Law Review: 2019, 52 :3 ,2. https://ideaexchange.uakron.edu/akronlawreview/vol52/iss3/2.
- Doyle, Kathryn. “IP Fundamentals-What Every CEO Should Know About IP in Biotechnology.” Journal of Commercial Biotechnology, 2019, 24.4.
- Coruzzi, L.A. Patents benefit patients and patent reform would spur diagnostic and therapeutic development. Nat Biotechnol 2022, 40:1178–1180 https://doi.org/10.1038/s41587-022-01405-z.
- Keswani, Chetan, and Cristina Possas, eds. Intellectual Property Issues in Life Sciences: Disputes and Controversies. CRC Press, 2024.
- Kumar, Nishant, Reetu Gour, and Navneet Sharma. “Intellectual Property Rights and Economical Development: A Brief Overview.” Journal of Scientific Research and Reports, 2024, 30.5: 145-162.
#BiotechStartups | #IPStrategies | #ScienceStartups | #Patents
Paperwork is your friend: Intellectual Property & Patents Part 1: Elements of a Robust Patent provides the information and steps needed to effectively set up and protect your IP.






One Comment